Gaucher
Gaucher disease (GD) is a rare, autosomal recessive disorder.1 Although rare, it is the most common type of lysosomal storage disease.1
Patients with GD have a deficiency of the enzyme glucocerebrosidase, the enzyme responsible for catalyzing glucocerebroside.1–3 The progressive accumulation of glucocerebroside throughout the body leads to a variety of symptoms that present in various organs.1,4–6
What is Type 1 Gaucher
Gaucher disease (GD) is a rare, autosomal recessive lysosomal storage disorder, in which deficiency of the enzyme glucocerebrosidase leads to the accumulation of its substrate glucocerebroside throughout the body, primarily in the spleen, liver, and bone marrow.1–3 The accumulation of glucocerebroside in the different areas of the body leads to the progressive, multi-systemic, and heterogeneous nature of the disease.1,4
There are three types of GD. Type 1 Gaucher disease (GD1) is the most prevalent form of the disease, accounting for more than 90% of all cases.4
- Unlike type 2 and 3 GD, GD1 is nonneuronopathic.4
- Individuals with GD1 can begin to exhibit symptoms at any age, including during early childhood and late adulthood.3
- The type and severity of symptoms will also vary among patients, and there can be varying degrees of involvement presenting in different organs in the same individual.2-4 This can be related to the rate of substrate accumulation.3,5,6
- The clinical course and life expectancy for patients with GD1 are also variable. While untreated patients will generally survive to adulthood, early recognition, diagnosis, and treatment of GD1 are key for the best possible patient outcomes and quality of life.1,4,7
- An early clinical presentation predicts a more severe disease course, highlighting the importance of recognizing GD1 as soon as possible to help ensure earlier intervention and inform treatment decisions.7
 Healthy Cell
Gaucher Cell
Healthy Cell
Gaucher Cell
Pathophysiology
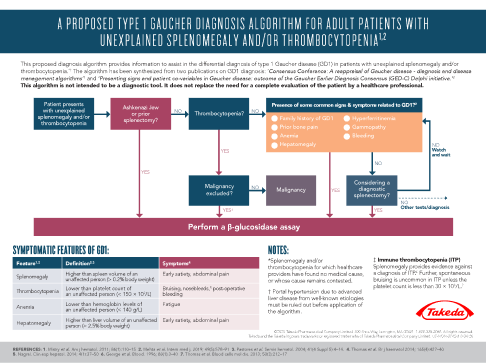
GD1 is a lysosomal storage disorder characterized by a deficiency of the enzyme glucocerebrosidase.2 This is caused by mutations of the gene that controls production of this enzyme.3
Without sufficient glucocerebrosidase, its substrate glucocerebroside accumulates within the lysosomes of macrophages, leading to the formation of ‘Gaucher cells’.1,4
‘Gaucher cells’ are engorged macrophages, visually characterized by a displaced nucleus and a lysosomal architecture that is distorted from its normally spherical shape.1,4,8
- Gaucher cells infiltrate organs and tissues enriched in cells of the mononuclear phagocyte system, e.g., the spleen, liver, and bone marrow.1,9,10
- Over time, they displace normal cells resulting in progressive impairment; the liver and spleen may become enlarged, which can interfere with normal functioning and cause a painful and swollen abdomen.1,8,9,11
- Displacement of hematopoiesis by Gaucher cell accumulation within the bone marrow can lead to anemia and thrombocytopenia, which in turn result in bleeding problems and can compromise the strength of the skeletal system.6,11

Prevalence, Genetics, and Inheritance
Both sexes can be affected by GD1, with males and females sharing an equal risk.3 GD1 is a pan-ethnic condition, although it does have a higher prevalence in people of Ashkenazi Jewish ancestry.5
GD1 affects:
- 1–9 in 100,000 within the overall population12
- ~1 in 600 within the Ashkenazi Jewish population3
- ~1 in 17 within the Ashkenazi Jewish community that are carriers13
- an estimated 6,000 individuals in the United States3,14
- GD1 is a lysosomal storage disorder and is caused by mutations of the GBA1 gene that controls the production of the glucocerebrosidase enzyme.14-16
- To date, more than 450 mutations in GBA1 have been identified.17
- The type of mutation may to some extent determine the type of GD, but there are other factors that affect the phenotypic expression. This has been demonstrated by cases in which symptom type, severity, and disease course have varied among siblings, even identical twins.6,18
GD1 is genetic and has an autosomal recessive inheritance pattern.3 If both parents are carriers, there is a 50% chance of the child being a carrier, a 25% chance of them having Gaucher disease, and a 25% chance that they will not be affected.3
Pedigree Diagram Illustrating the Autosomal Recessive Inheritance Pattern of Gaucher Disease

The age of onset and the severity of symptoms of type 1 Gaucher disease (GD1) can vary considerably.1,2 The main clinical manifestations include:
Visceral

Splenomegaly occurs in 95% of patients with GD1. It may be one of the first and most physically prominent presentations of GD1 in patients. Splenomegaly results in abdominal distension and early satiety.4–7 Many patients complain of acute abdominal pain, which can be worsened by splenic infarction.1,8
Enlargement of the liver occurs in 80% of patients with GD1, also contributing to abdominal pain and early satiety.1,5,7 Hepatomegaly may progress to liver disease and cirrhosis.1,4
Hematological

Bruising, bleeding, and nosebleeds occur due to thrombocytopenia.1,4,6
Fatigue is common, occurring in 50% of patients with GD1.6,7
Gammopathy can be polyclonal or monoclonal, and is detected very often in patients.9,10 This can lead to long-term hematological complications.9
Hyperferritinemia is very often detected in patients and can contribute to long-term hematological complications.10
Long-term hematological complications include a risk of developing hematological malignancies and solid tumors.10,11
Skeletal

Skeletal involvement occurs in 70–100% of patients with GD1 and includes abnormal bone shaping, bone pain, bone infections, and fractures.5 Skeletal manifestations are associated with considerable pain, limitations in mobility, and a significantly reduced quality of life.11,12 In severe cases, they can lead to potentially irreversible complications or long-term disability.13
This summarizes the key clinical manifestations of GD1; however, a GD1 diagnosis and the resulting symptoms patients experience can also lead to further, long-term impacts on patients that require ongoing Monitoring and Management. Visit the Further Impact section to learn more.

What to Do If You Recognize the Symptoms of GD1
Laboratory testing for Gaucher disease (GD) should be carried out when a patient presents with signs indicative of the disease, such as unexplained splenomegaly, anemia, or thrombocytopenia.14 Suspicion should be higher when the patient is of Ashkenazi Jewish descent.13,14 If GD is suspected, an enzyme assay as well as genetic testing should be performed to confirm the diagnosis. Learn more about the definitive tests for GD1 here.

Overview
A diagnosis of type 1 Gaucher disease (GD1) should be confirmed with an enzyme assay to determine levels of β-glucosidase enzyme activity, and genetic testing to identify mutations that cause the disease.1
A bone marrow biopsy or smear can detect Gaucher cells, but it is not recommended as a diagnostic tool, as it is not differential. Furthermore, it is an invasive procedure, and there is a risk of complications.1–3
Some states (including Missouri, New York, and Illinois) currently offer genetic testing to screen newborns for Gaucher disease (GD), with further states currently considering implementation of this testing.4 Check the current status of newborn screening for GD in your state in case your patients have questions or need guidance in this area.

Testing
The β-glucosidase enzyme assay measures the levels of the enzyme glucocerebrosidase.5 A GD diagnosis is confirmed by establishing glucocerebrosidase enzyme activity in mononuclear cells, leukocytes, or cultured fibroblasts. These are obtained by either a skin biopsy or dried blood spot test.6–8 Low levels of this enzyme, less than 15% of mean normal activity, indicate a diagnosis of GD.6,9 Enzyme activity, however, does not predict disease severity.1,6
Genetic testing requires either a blood or saliva sample, used to extract the patient’s DNA.10 Genetic sequencing can then be used to detect specific mutations in the GBA1 gene that result in GD.11,12
This algorithm* provides information to assist in the differential diagnosis of GD1 in patients with unexplained splenomegaly and/or thrombocytopenia.
This algorithm is not intended to be a diagnostic tool. It does not replace the need for a complete evaluation of the patient by a healthcare professional.
In cases of splenomegaly and/or thrombocytopenia it is important to include GD1 in differential diagnoses in order to avoid potentially harmful splenectomy or irreversible complications.7,15
*This algorithm has been synthesized from two publications on type 1 Gaucher disease diagnosis: ‘Consensus Conference: A reappraisal of Gaucher disease – diagnosis and disease management algorithms’ and ‘Presenting signs and patient covariables in Gaucher disease: outcome of the Gaucher Earlier Diagnosis Consensus (GED-C) Delphi initiative.’3,13
**This diagnostic algorithm for pediatric patients has been synthesized from two publications on type 1 Gaucher disease diagnosis: ‘The diagnosis and management of Gaucher disease in pediatric patients: Where do we go from here?’ and ‘Screening, patient identification, evaluation, and treatment in patients with Gaucher disease: Results from a Delphi consensus.’6,14

Diagnostic Challenges and Screening
Due to the rare nature and heterogeneous presentation of GD1, patients often consult several different specialists before reaching the correct diagnosis.16,17 Although clinical presentation of variable severity occurs in childhood for the majority of patients, diagnosis is often delayed 10–15 years until patients are in adulthood.15,17 Patients are often misdiagnosed, which can lead to disease complications, persistence of untreated symptoms, emotional distress, and frustration and depression at the lack of an explanation for their symptoms.6
-
One of the key reasons for misdiagnoses is that GD1 symptoms can overlap with a number of
other disorders, such as:
- Visceral: liver cancer6
- Hematological: autoimmune hemolytic anemia; hematological malignancies; bleeding disorders; idiopathic thrombocytopenia purpura3,4,18,19
- Skeletal: synovitis or osteomyelitis; multiple myeloma6,9
Additional diagnostic challenges of GD1, which may lead to delayed diagnoses or misdiagnoses, include:
- the high phenotypic heterogeneity in the initial presentation, which can make GD1 difficult to recognize6,17
- low physician awareness of GD, due to its rarity5,16
- the existence/appearance of nonspecific and mild symptoms of GD1, which can mean that physicians do not always consider it in their differential diagnoses2,6,15
Genetic testing can also be used to identify carriers of GD1. Although carriers do not experience symptoms of the disease, it is important that they are identified because GD is a hereditary condition.12
Carrier screening for mutations commonly associated with GD1 is recommended for the Ashkenazi Jewish population and other individuals who are at high risk for the disease.6,20
Testing must be offered in conjunction with genetic counseling to provide couples at risk, even asymptomatic individuals, with a description of the range of associated phenotypes and their options, which include prenatal diagnosis.9
Due to its hereditary nature, an additional challenge faced by patients, carriers, and their families is how to approach family planning. Make sure your patients affected by GD1 know they are not alone in this and are directed toward any available Resources or referred to appropriate specialists to help them with these decisions.

Importance of Early Recognition and Diagnosis
Prompt diagnosis of GD1 is essential because the disease is progressive.5 Delayed recognition and subsequent delayed diagnosis of the disease lead to late management, which can lead to irreversible complications, such as avascular necrosis or multiple myeloma.8,15 Therefore, the aim should be to recognize, diagnose, and treat before the development of such irreversible complications.2 Early recognition can reduce unnecessary investigations and the time to diagnosis, and therefore lead to more effective management strategies and the timely initiation of appropriate treatment.5,16 This can help to alleviate some of the anxiety experienced by patients and their families, and is therefore critical to ensuring the best patient outcomes.16 Regular Monitoring and Management are required for patients with GD1 following their diagnosis.7,8

Monitoring
Patients with type 1 Gaucher disease (GD1) should be regularly monitored using blood tests, measurement of visceral volumes (spleen and liver), and skeletal scans to detect any complications that can occur as the disease progresses.1,2 The monitoring interval, which is usually every 6–24 months, depends on the recommendations of the healthcare provider, the patient’s individual experience with the disease, and/or treatment.1
Blood tests are often used to monitor disease progression and measure a patient’s:1,3
- Hemoglobin count
- Platelet count
Additionally, the serum levels of several biological markers indicate disease severity and progression:1,3,4
- Glucosylsphingosine (lyso-Gb1/lyso-GL1)2
- Tartrate-resistant acid phosphatase (TRAP)1
- Chitotriosidase (CHIT1)1,3,4
- Angiotensin-converting enzyme (ACE)1
- Chemokine ligand 18 (CCL-18)2,3
Visceral volumes can be assessed with:1,3
- Volumetric MRI (since repeat assessment is routine in Gaucher disease)1,3
- CT or ultrasound can be used when MRI is unavailable1,3
Skeletal scans are used to detect bone marrow infiltration and bone disease, and can be assessed using:1
- MRI, which is particularly useful to identify bone infarction and necrosis
- Dual-energy X-ray absorptiometry (DEXA) is the gold standard method for assessing bone mineral density

Management and Treatment Options
As GD1 is a multi-systemic and heterogeneous disorder, a multidisciplinary team and individualized approach is required for optimal management.5,6 First, the team should conduct a comprehensive assessment of all possible disease manifestations.7 Therapeutic goals should then be established for the individual patient based on each disease manifestation.7 Achievement and maintenance of these goals rely on regular ongoing monitoring and the adjustment of therapy when goals are not reached.4,7 Such management is designed to provide personalized supportive care for the patient, considering the patient’s severity and rate of disease progression, and their quality of life.6–8
Healthcare providers can work with patients to help manage their GD1 symptoms. In addition, treatments are available for GD1:9
- Enzyme replacement therapy (ERT) infusion – a functional version of the deficient enzyme is administered by intravenous infusion to metabolize glucocerebroside10,11
- Oral treatment with a substrate reduction therapy (SRT) – inhibits the synthesis of the substrate glucocerebroside10,11
ERT is the only currently approved treatment for pediatric patients with type 1 Gaucher disease.5 Regular ERT infusions are recommended for all children and adolescents who are symptomatic of GD1 and should be initiated as soon as possible after diagnosis to help prevent irreversible complications.5

Supportive Therapies
Management of GD1 has, and occasionally continues to be, limited to supportive care with transfusions, splenectomy, and orthopedic surgery.12 Transfusions may be given in cases of severe anemia or bleeding, for example, prior to invasive procedures, during pregnancy, and postpartum hemorrhage.5,8,11 Some patients may become transfusion dependent.11
Painful bone crises often require temporary immobilization, and analgesics may be given to relieve pain.2,8 Bisphosphonates can be used, but patients receiving them should be monitored for osteonecrosis of the jaw.8 The use of bisphosphonates is controversial in GD1, as the pathophysiology of bone-mass decline is poorly understood.2
Joint replacement can be considered for restoration of function and relief from chronic pain.5 Orthopedic surgeries may be required for bone complications, including avascular necrosis and pathological fractures.2
Historically and even at times today, partial or total splenectomy is performed in the presence of hypersplenism. While effective, this procedure increases the risk of infections, avascular necrosis, and pulmonary hypertension. Furthermore, splenectomized patients generally suffer from increased bone and liver deposits, leading to increased bone complications and susceptibility to infection. Splenectomy should therefore be avoided and only performed in exceptional circumstances, such as emergency situations.2,8,9,14
GD1 will affect a patient physically but also can contribute toward emotional and psychological issues. For this reason, psychological support should be offered to patients to help reduce this impact of the disease on patients and their families. Learn more about the potential psychological and psychosocial impacts of GD1 here. It is also important that patients and their loved ones be made aware of patients’ associations that they can connect with.2,5,8 Visit our Resources page to learn about organizations currently working to improve understanding of GD1 and provide support to patients living with this rare condition.

FURTHER IMPACT - DISEASE PROGRESSION
For younger patients with GD1, delayed growth and puberty can often arise as complications in the disease course.2,4,5
- Around half of younger patients with GD exhibit delayed growth, with approximately 25% having a shorter stature than expected when compared with midparental height.7
- Patients who exhibit significantly stunted growth during childhood are likely to have severe visceral involvement and can also experience delayed puberty.7
- Growth below the 5th percentile is seen in approximately 34% of children with GD1 specifically.2
- Children with a more severe form of GD1 also display delayed milestones and failure to thrive.11
- For children diagnosed with GD1, regular monitoring of growth should be performed at regular intervals of every 3 or 6 months.11,15
- With appropriate treatment and management, children with GD experiencing delayed growth can achieve normal height, when compared with population averages.7
As a result of the variable clinical course seen in GD1 patients, life expectancy can be highly variable.7
- GD1 patients can fall anywhere on a spectrum between a severe form of the disease with onset in early childhood to an indolent or even asymptomatic disorder presenting in elderly adults.7
- The average life expectancy of GD1 patients has been reported to be approximately a decade shorter than that of the general population; however, it has been acknowledged that the positive effects of appropriate, modern management and treatment have not yet been taken into account.5

FURTHER IMPACT - WELLBEING
HRQoL of patients can be impacted by many aspects of GD1, including the range and severity of symptoms, as well as how they interact with aspects of daily life.16
- The HRQoL of younger patients specifically is seen to be negatively impacted by many of the key symptoms associated with GD1 – most notably bone, joint, and abdominal pain, as well as chronic fatigue and mobility limitations.4,5,16
- Subjective experiences relating to symptoms, for example feeling ill, sad, and unable to be involved in various activities, were also reported by children to impact substantially on their perceived quality of life, especially in relation to social and school functioning.16
- Studies comparing adult GD patients to the general population have found that HRQoL domains (e.g., physical functioning, presence of physical limitations, pain, general state of health, vitality, and social functioning) are significantly lower in patients.16
- With appropriate management, which also takes into account psychological and subjective emotional functioning, patients may perceive improvements in HRQoL; for example, they may be able to better control their most debilitating symptoms, lead more active and socially fulfilling lives, and pursue their education or careers.9,16
Receiving a diagnosis of a chronic, lifelong condition and living with its symptoms can lead to various psychological and psychosocial issues.17 There can be specific considerations for different age groups, including what each perceives as the most prevalent issues they face when living with GD1.
Younger children:
- It is vital to consider the implications of receiving such a significant diagnosis at a very young age; children who have grown up with a GD diagnosis may progress into adulthood without a proper understanding of the implications of their condition or the importance of treatment.5
- Additionally, children may experience considerable psychosocial problems, for example difficulty adjusting to life with their condition, as well as feelings of distress, including anger, denial, fear, insecurity, and isolation.16
Older children and adolescents:
- Delayed growth and puberty can have a negative effect on the body image and social functioning of adolescent patients, leading to self-stigmatization and decreased self-esteem.16
- An increasing awareness of ‘being different’ can be amplified when symptoms (e.g., fatigue, splenomegaly, or bone disease) prevent patients from participating in physical or social activities, such as sports.16
- School performance may also be affected by symptoms such as chronic pain and fatigue.16
- Older children and adolescent patients can benefit from sensitive genetic counseling for their condition and support for the above issues, as well as support for the transition from pediatric to adult healthcare.5
Adults:
- Some of the domains affected by GD1 in adult patients include difficulties coping with the diagnosis and physical functioning-related daily activities at school, home, or work.16
- Patients find that their social lives, including capacity for recreational activities are limited by both a lack of energy and pain.16
- Stress associated with treatment burden, time consumption, and cost is often reported.16
- Patients perceive GD1 as likely to limit their options in life and impact future plans; their emotional health is impacted by these limitations, causing feelings of low self-esteem, anger, anxiety and depression.16
- Psychological counseling can therefore be beneficial for all patients with GD and their families, given the chronic, lifelong nature of the disease, presence of incapacitating symptoms, and effects of these on both day-to-day functioning and overall outlook on life.11,16

Downloadable Materials
The following resources might be useful in your clinical practice to provide further information on managing patients with type 1 Gaucher disease (GD1):

Use this algorithm to learn more about the common features of GD1 and help diagnose adult patients with unexplained splenomegaly and/or thrombocytopenia.

Use this algorithm to learn more about the common features of GD1 specific to children and adolescents and help diagnose pediatric patients with unexplained splenomegaly with thrombocytopenia and/or anemia.

This flyer provides guidelines for the clinical monitoring of adult patients diagnosed with type 1 Gaucher disease and highlights the benefits of ongoing monitoring.

This brochure outlines three steps toward a better understanding of type 1 Gaucher disease, with the intention of spreading awareness of GD1.
There are also resources available that you can share with your patients to help them on their journey with GD1:

This caregiver guide, for anyone caring for a patient with GD1, is available for download below in both English and Spanish.

Other Resources
There are a number of organizations that specialize in raising GD1 and wider rare-disease awareness and building communities for patients living with these diseases. We encourage you to utilize the information and resources offered by these organizations and to make your patients aware of the additional support that they offer.

The NGF supports physicians in their efforts to better understand the diagnosis and treatment of Gaucher disease. Information and resources can be found on their website. Resources for your patients and their families are also provided.

The GCA is a patient organization that aims to support the patient community. Resources and opportunities to connect with other patients are available for your patients.
NORD provides resources for clinicians about specific rare disorders, including type 1 Gaucher disease, to facilitate the timely diagnosis and treatment of their patients. NORD also provides programs and services to support your patients.

My Life with Gaucher provides a space where people can read about the experiences of those with type 1 Gaucher disease. Here you can find advice from healthcare professionals in the field as well as stories from real patients living with GD1.

CheckRare is the only publishing and learning platform dedicated exclusively to rare diseases and cancers. Visit to find original content from a range of perspectives, including patients and healthcare professionals, as well as industry and regulatory bodies.

Thank you
for signing up to the mailing list.
To unsubscribe, please click here.
Stay updated
Sign up for news and information on type 1 Gaucher disease.
* Required field

SITEMAP
©2023 Takeda Pharmaceuticals U.S.A., Inc., 300 Shire Way, Lexington, MA 02421. 1‑877‑TAKEDA‑7 (1‑877‑825‑3327). All rights reserved. TAKEDA® and the TAKEDA Logo® are registered trademarks of Takeda Pharmaceutical Company Limited. US‑NON‑8044v1.0 12/23
All images used on this website are stock photography images and do not portray actual patients.


Hematologist

Geneticist
 Healthy Cell
Gaucher Cell
Healthy Cell
Gaucher Cell